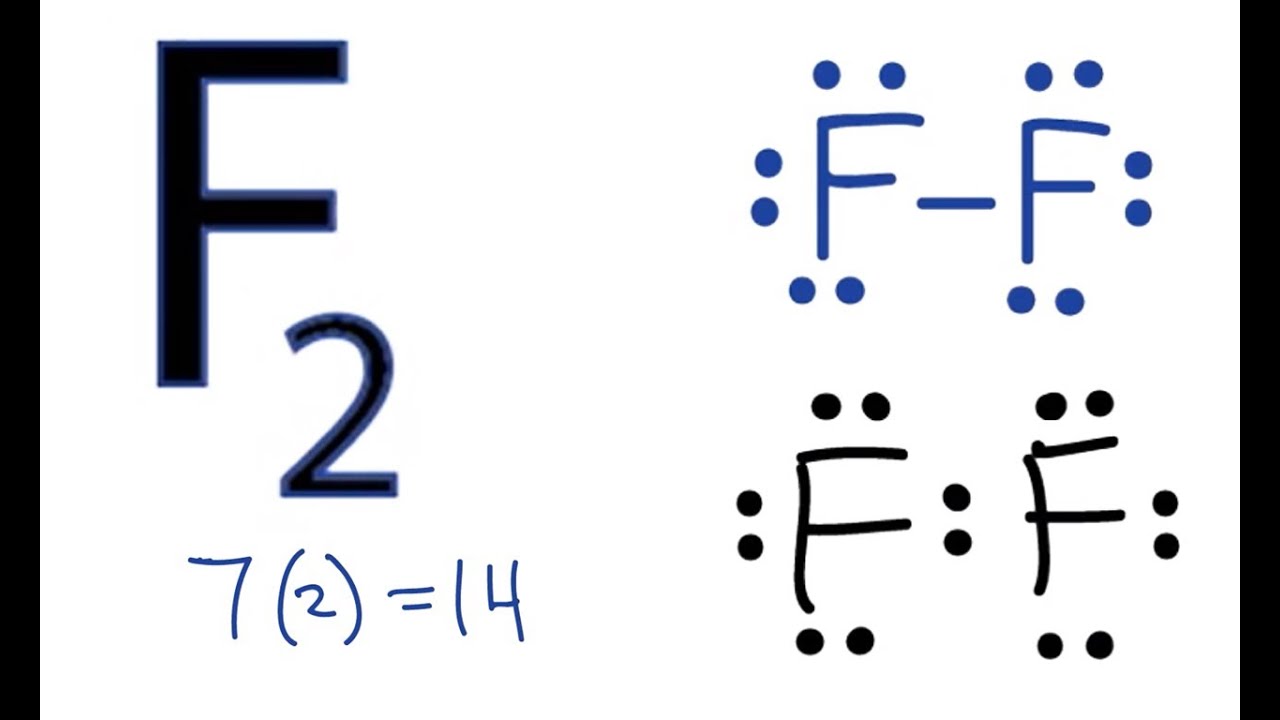Lewis Structure Of 2 Hf Molecules
Get more help from chegg.
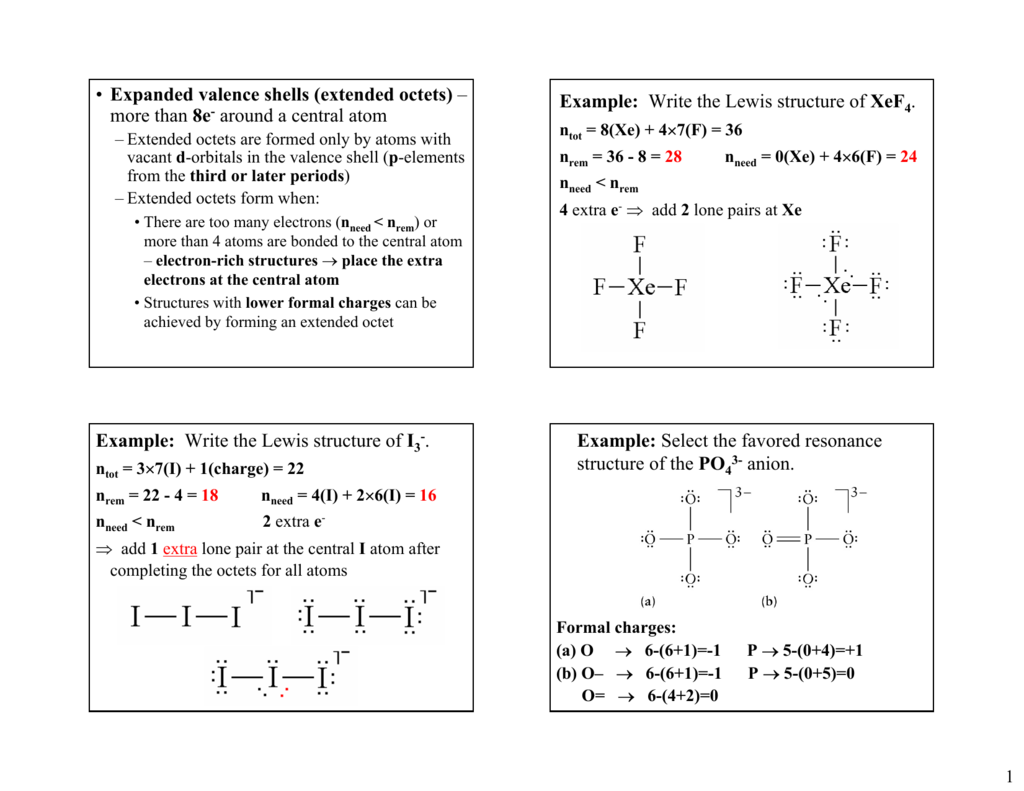
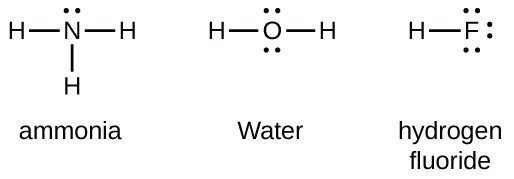
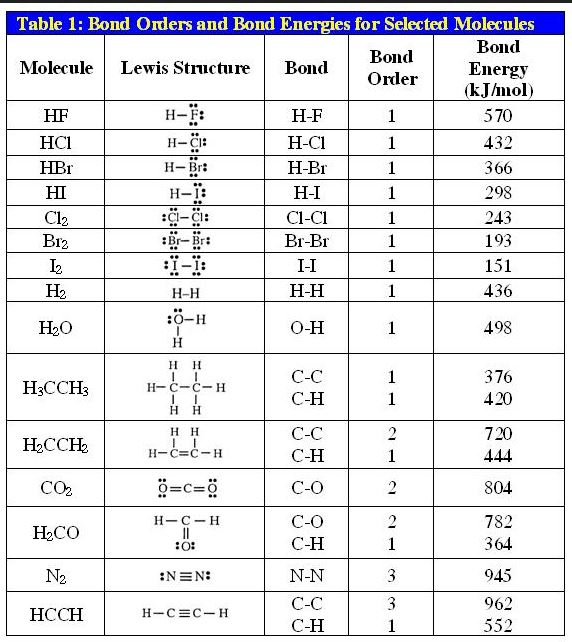
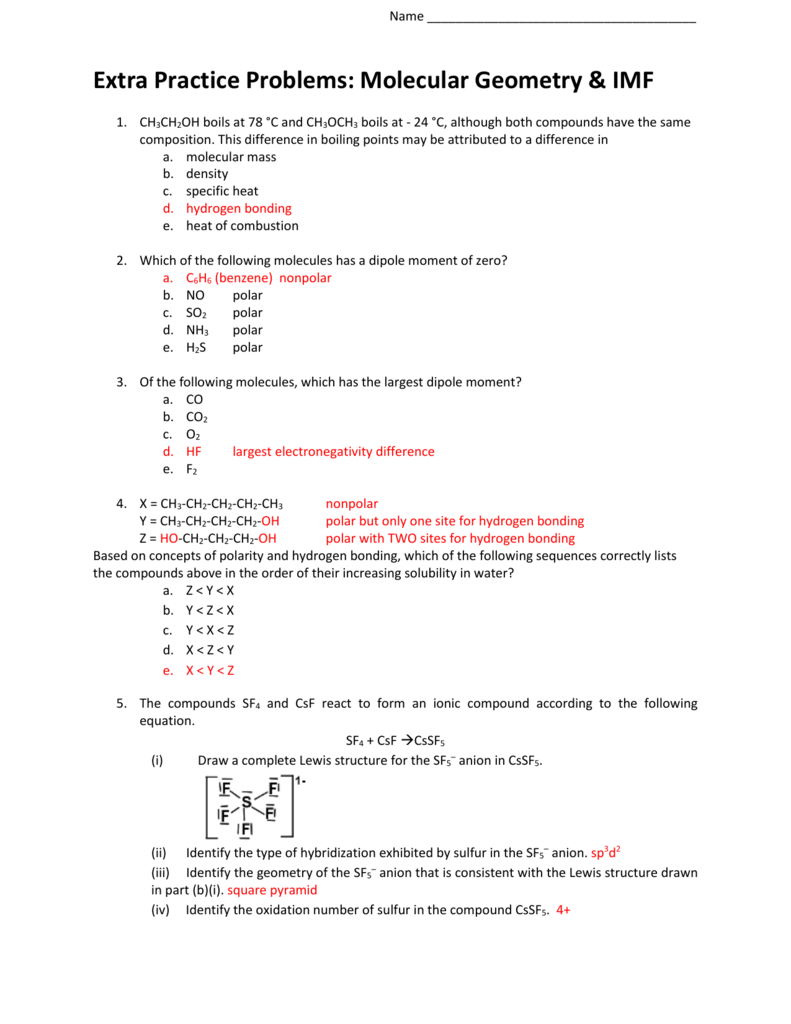
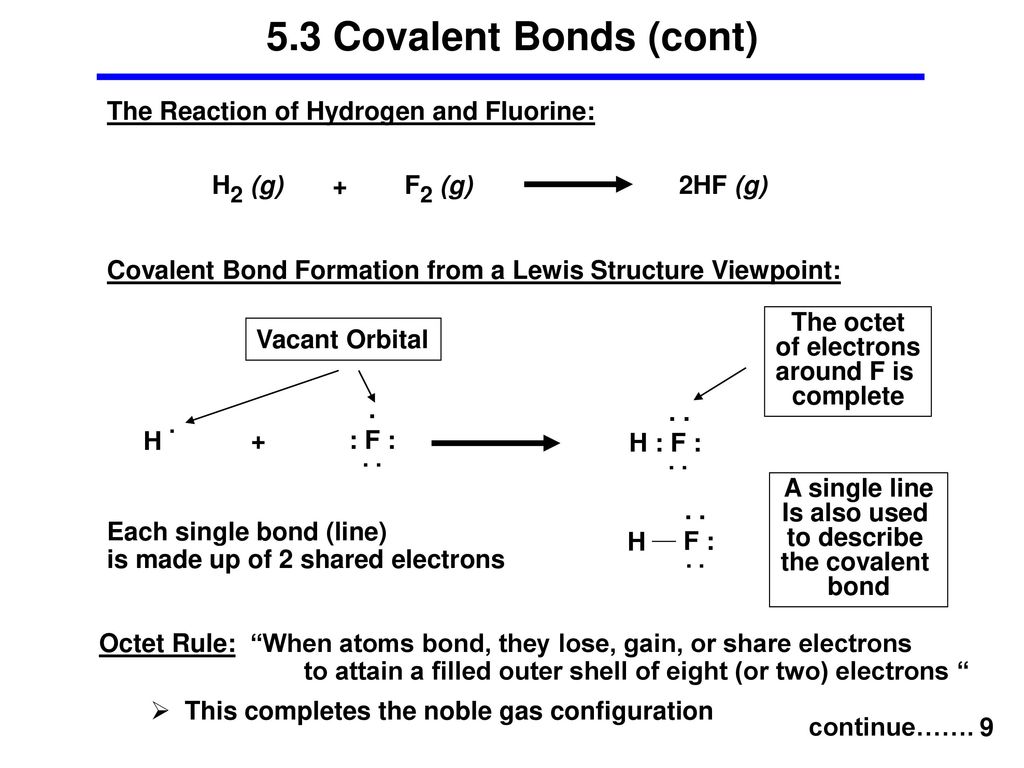
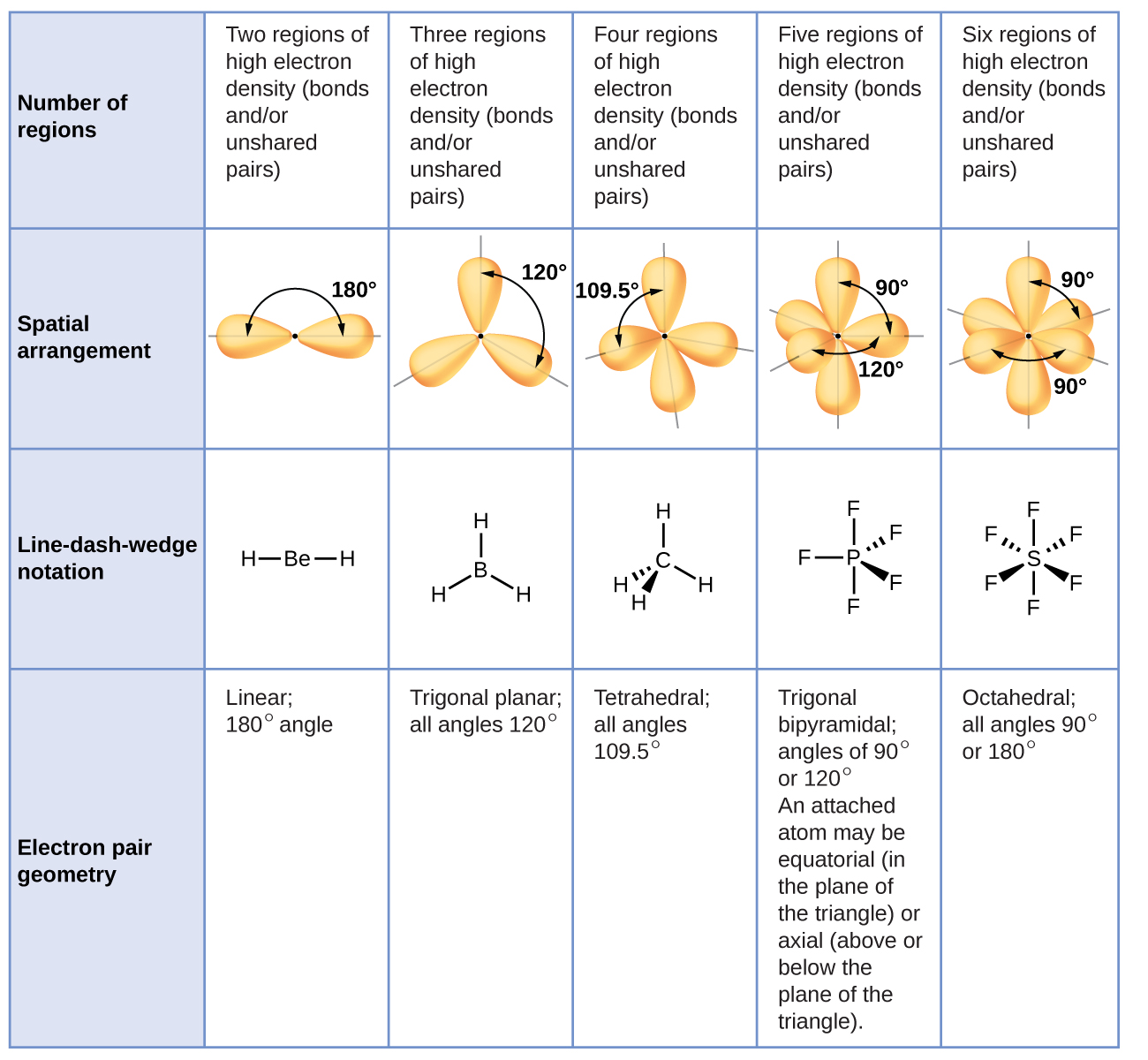
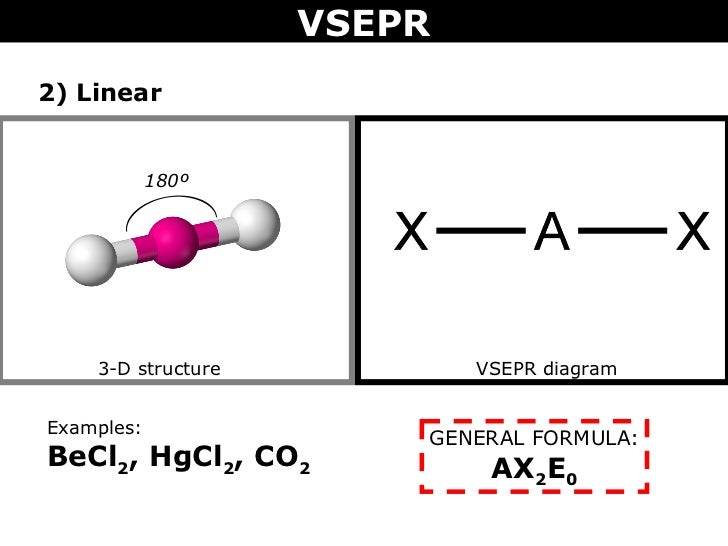
Lewis structure of 2 hf molecules. The structure on the right is the lewis electron structure or lewis structure for h 2 o. Draw a lewis structure to illustrate hydrogen bonding between 2 molecules of hf. Teflon hf is widely used in the petrochemical industry as a component of. Steps for writing lewis structures.
The hydrogen bond should be drawn as 4 heavy dots in the correct location. Determine the total number of valence electrons in the molecule or ion. A complex ion consists of a central atom typically a transition metal cation surrounded by ions or molecules called ligands these ligands can be neutral molecules like h 2 o or nh 3 or ions such as cn or oh often the ligands act as lewis bases donating a pair of electrons to the central atom. Each hydrogen atom group 1 has one valence electron carbon group 14 has 4 valence electrons and oxygen group 16 has 6 valence electrons for a total of 2 1 4 6 12 valence electrons.
In lewis structures you try to ensure each atom has filled its octet with the exception of hydrogen which is stable with 2 electrons.